38 orbital diagram for ti2+
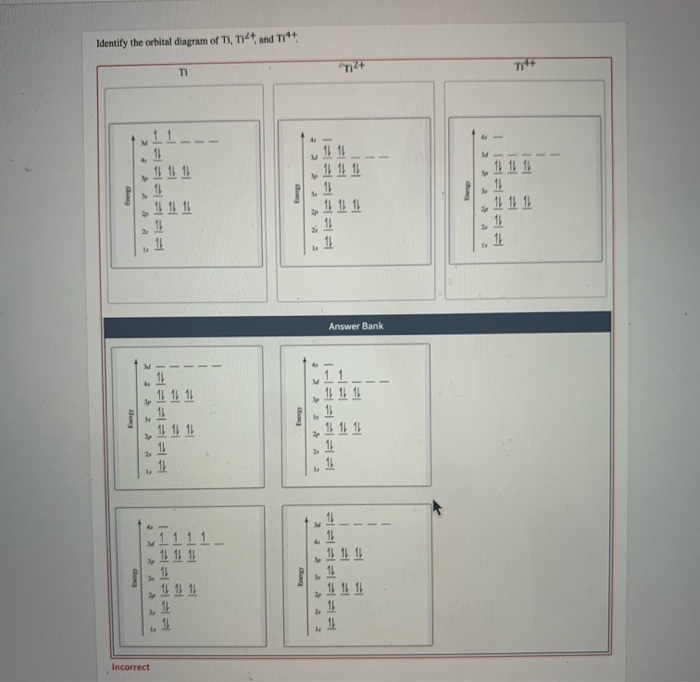
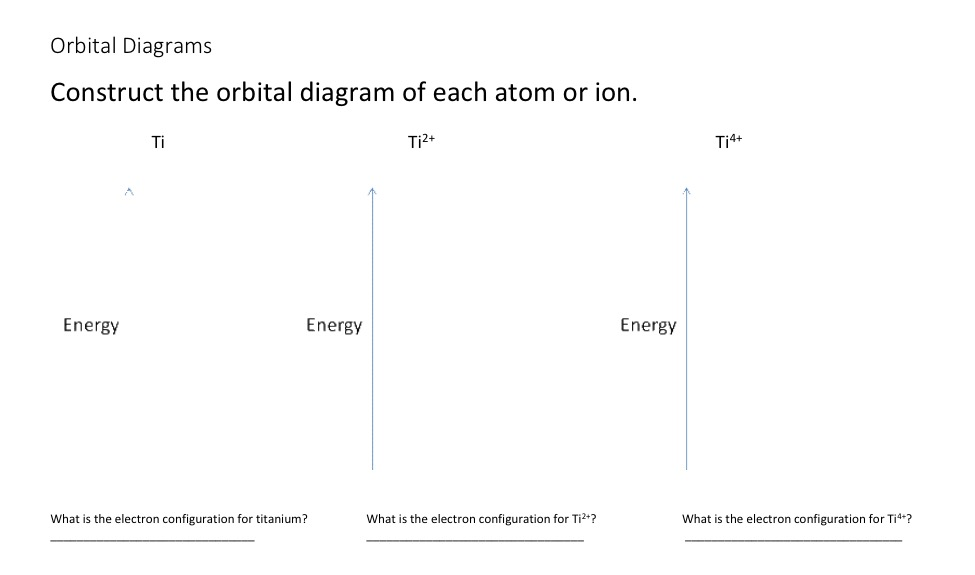
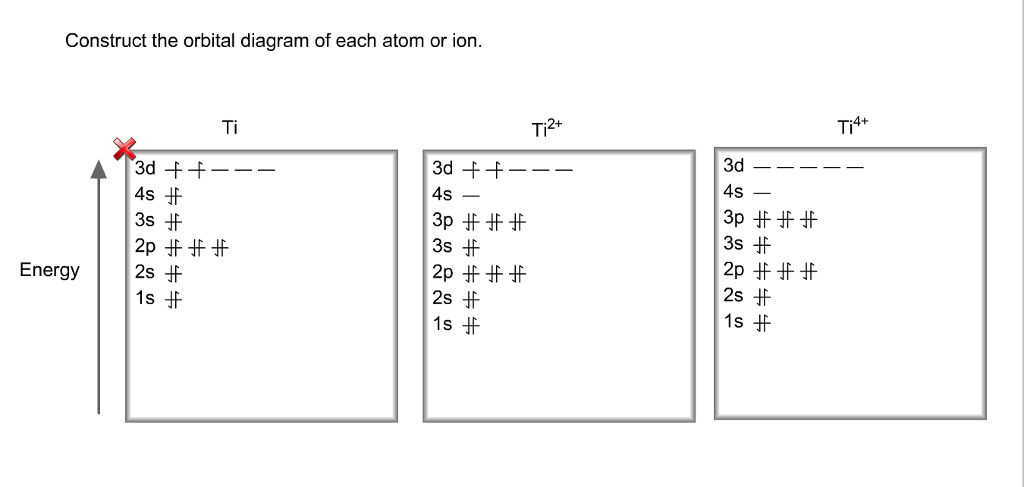
Orbital Diagram For Ti2 — UNTPIK APPS Orbital Diagram for Ti2. electron configuration for the titanium ion ti2 this video shows you how to write the electron configuration for the titanium ion ti 2 electron orbitals question 111 so if you count the electrons of the ti2 in the s orbital you need to know hund s rule and specifically this diagram electron orbitals OneClass: What is the orbital diagram of each atom or ion ... greenkangaroo798 Lv1. 28 Nov 2020. What is the orbital diagram of each atom or ion? Ti, Ti 2+, Ti 4+. Answer. + 20. Watch. 1. answer.
37 orbital diagram of ti - Wiring Diagram Images Solved Identify the orbital diagram of Ti, Ti2+, and Ti4+ ... I've been tasked with drawing rhe MO diagram for Sulfure Oxide and I'm not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy ...

Orbital diagram for ti2+
Electron configuration of ti4 | electron configuration for ... Construct the orbital diagram of each atom or ion. Ti. Ti 2 Oct 15, · Normally you would expect the electron configuration of Ti to be [Ar] 4s23d2, but in actuality it is 4s13d3. If you took two electrons away due to the +2 charge, you would have 4s13d1. Titanium | Ti (Element) - PubChem Titanium is the ninth most abundant element in the earth's crust and is primarily found in the minerals rutile (TiO 2 ), ilmenite (FeTiO 3) and sphene (CaTiSiO 5 ). Titanium makes up about 0.57% of the earth's crust. Jefferson Lab, U.S. Department of Energy. From the Latin titans, the first sons of the Earth, Greek mythology. Molecular orbital diagram of TiO2, Ti2O3, TiO. Reproduced ... In the rutile IrO 2 , the site symmetry of Ir is D 2h , where the e g level is split into the a 1g and b 1g levels (σ-symmetry) and the t 2g level is split into the e g and b 2g levels (π-symmetry).
Orbital diagram for ti2+. WRite the electronic configuration of ti4+ ion - Brainly.in As the atomic number of Titanium is 22, it has 22 electrons. So the electronic configuration of titanium is . As is electronic configuration of Nobel gas argon, it can be represented as [Ar]. means that the titanium ion has lost four electrons and thus its electronic configuration will be [Ar]. Construct the orbital diagram of each atom... | Clutch Prep Problem: Construct the orbital diagram of each atom or ion.TiTi2+Ti4+ FREE Expert Solution Show answer Answer: 91% (381 ratings) Sign up for free to keep watching this solution Sign up for free. 584,686. students enrolled. 97%. improved grades. 2,784. minutes of videos. Orbital Diagram Of Ti2+ - Wiring Diagrams Orbital Diagram Of Ti2+ Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+. Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . Titanium(Ti) electron configuration and orbital diagram Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2. For example, 1. n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2 electrons. 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8 electrons. 3. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. 4. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 42= 32 electrons. Therefore, the maximum electron holding capacity in the first shell...
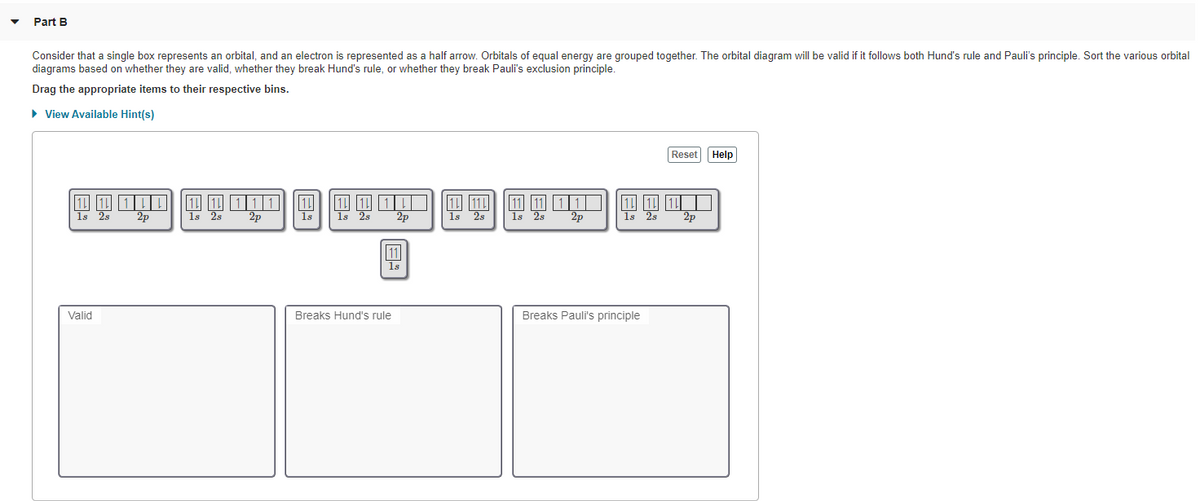
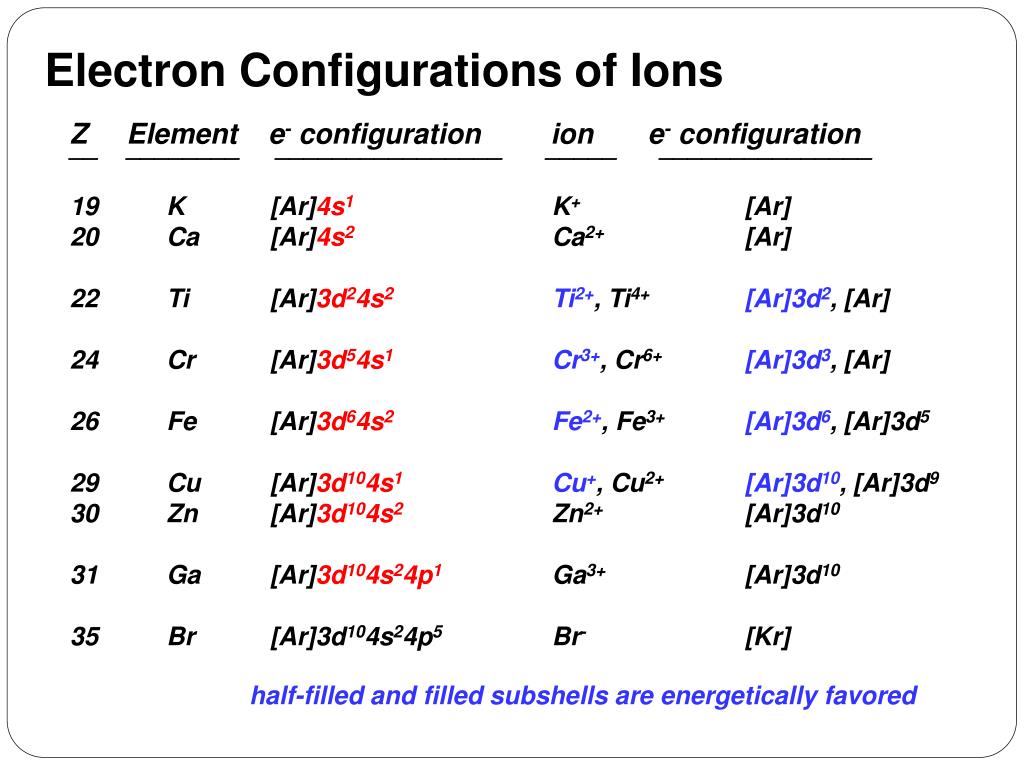
The electronic configuration of titanium ion (Ti2+) is - Vedantu The electronic configuration of titanium ion (Ti2+) is: A. (Ar)3d2 4s2. B. (Ar)3d2. C. (Ar)3d2 4s1. D. (Ar)4d1. Verified. 157.2k+ views. Exam 2 Flashcards - Quizlet Choose the ground state electron configuration for Ti2+ ... Choose the valence electron orbital diagram that represents the ground state of Se2-D (#1) The solid compound K2S2O3 contains. K+ ions and S2O3^2-Of the following, which atom has the largest atomic radius? K. Choose the best Lewis structure for SF4. E (#28) Orbital Diagram of Titanium (Ti), electron configuration ... This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble... Electronic Structure of Atoms (Electron Configurations ... The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
What is the electron configuration of Ti plus4? - Answers The electron configuration is the number of electrons in each energy level of an element. The electron configuration of Li is, 1s2 2s1. The electron configuration of F is, 1s2 2s2 2p5. Orbital Diagram For Ti2+ Jun 12, 2018 · on Orbital Diagram For Ti2+. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? The probability of finding an electron at the nucleus is 0. However, once the 4s orbital is filled, it becomes higher in energy than the 3d orbitals. This means that when titanium loses electrons, it does so. Orbital Diagram Of Ti2+ - schematron.org Aug 14, 2018 · Oct 15, · Normally you would expect the electron configuration of Ti to be [Ar] 4s23d2, but in actuality it is 4s13d3. If you took two electrons away due to the +2 charge, you would have 4s13d1. So if you count the electrons of the Ti2+ in the s orbital only, you have 1 from each of H, He, Li, Be, Na, Mg, and K. The electron configuration of a neutral titanium atom is. Ti: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2. The two electrons that are lost when the Ti2+ is formed will come from the 4s orbital, which means that the electron configuration of the cation is Ti2+:1s 2 2s 2 2p 6 3s 2 3p 6 3d 2
What is the electron configuration of "Ti"^(2+)? | Socratic This means that when titanium loses electrons, it does so from the 4s orbital first. Ti: 1s22s22p63s23p63d24s2 Therefore, the two electrons that are lost when the Ti2+ is formed will come from the 4s orbital, which means that the electron configuration of the cation is
Solved Identify the orbital diagram of Ti, Ti2+, and Ti4 ... Answer to Solved Identify the orbital diagram of Ti, Ti2+, and Ti4+.
Construct the orbital diagram of each atom or ion.TiTi2+ - Chegg See the answer See the answer done loading. Construct the orbital diagram of each atom or ion. Ti. Ti2+. Ti4+. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
Electron Configuration Calculator - Find element configuration It also describes every electron as moving freely in an orbital, in an average field generated by other orbitals. For example, electron configuration of Phosphorus (P) is 1s^2 2s^2 2p^6 3s^2 3p^3. Usually, Physicists and chemists use the standard notation to refer to the electronic configurations of molecules and atoms. For atoms, the standard ...
Electron Configuration for Oxygen (O) - UMD Oxygen is the eighth element with a total of 8 electrons. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be ...
What is the orbital diagram for Ti 2+? I got 1s two arrows ... Nov 04, 2012 · What is the orbital diagram for Ti 2+? I got 1s two arrows, 2s two arrows, 2p 6 arrows, 3s two arrows, 3p six arrows, 4s two arrows but it is wrong. It said ions of d-block metals typically lack the outermost s electrons that are present in their neutral counterparts.
PDF 1.6 Term Symbols A brief general review of atomic ... Section 1.6 - 4 • A general term symbol that uniquely describes a specific electronic configuration looks like this: (2S+1)L J where 2S + 1 is the spin multiplicity (and S is the total spin angular momentum.) L is the total orbital angular momentum J is the total angular momentum (spin + orbital) S = 0 → "Singlet" S = ½ → "Doublet" S = 1 → "Triplet" etc.
What is the orbital diagram of TI? - AnswersToAll What is the correct orbital diagram for phosphorus? The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s22s22p63s23p3.
Konfigurasi Elektron Ion Ti3+ | ardra.biz Diagram Orbital Atom Nikel. Untuk menentukan electron yang tidak berpasangan cukup membuat diagram orbital dari subkulit yang diisi electron tidak penuh yaitu 3d yang terisi 8 elektron. Subkulit d terdiri 5 orbital yang dapat ditempati oleh 10 elektron maksimum. Jumlah elektron tidak berpasangan pada subkulit 3d dapat dilihat pada gambar berikut
Electron Configuration for Ti , Ti3+, and Ti4 ... - YouTube To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number...
Answers to Practice Test Questions 5 Electron - Chemistry ... Note that the 4s electrons are removed before the 3d electrons, so the electron configuration of Ti2+ would be [Ar]3d2 not [Ar]4s2. core electrons valence ...
Learnsmart Chapter 8 Part 1 and Part 2 Flashcards - Quizlet Which of the sublevels maybe utilized in constructing a partial orbital diagram for a Period 3 element? Select all that apply.-3p-3s. Which of the following is the correct condensed electron configuration for selenium (Z = 34)? ... Ti2+ : [Ar]3d2 Mn2+ : [Ar]3d5.
Electron Configurations for Transition ... - Brightstorm Now if it's Ti4+, now we've taken away two additional electrons compared to the Ti2+. So we would write out 1s2, 2s2, 2p6, 3s2, 3p6, and then the 3d2-electrons would be gone. So compared to the original Titanium atom, we gave away four electrons. So both the 4s2, and the 3d2 electrons would be gone.
Molecular orbital diagram of TiO2, Ti2O3, TiO. Reproduced ... In the rutile IrO 2 , the site symmetry of Ir is D 2h , where the e g level is split into the a 1g and b 1g levels (σ-symmetry) and the t 2g level is split into the e g and b 2g levels (π-symmetry).
Titanium | Ti (Element) - PubChem Titanium is the ninth most abundant element in the earth's crust and is primarily found in the minerals rutile (TiO 2 ), ilmenite (FeTiO 3) and sphene (CaTiSiO 5 ). Titanium makes up about 0.57% of the earth's crust. Jefferson Lab, U.S. Department of Energy. From the Latin titans, the first sons of the Earth, Greek mythology.
Electron configuration of ti4 | electron configuration for ... Construct the orbital diagram of each atom or ion. Ti. Ti 2 Oct 15, · Normally you would expect the electron configuration of Ti to be [Ar] 4s23d2, but in actuality it is 4s13d3. If you took two electrons away due to the +2 charge, you would have 4s13d1.



















![Chapter...D) [Kr]](https://s3.studylib.net/store/data/008758548_1-5cb6ca63160320ee288f5c0c2564d9db-768x994.png)



0 Response to "38 orbital diagram for ti2+"
Post a Comment