40 write orbital diagram for au+.
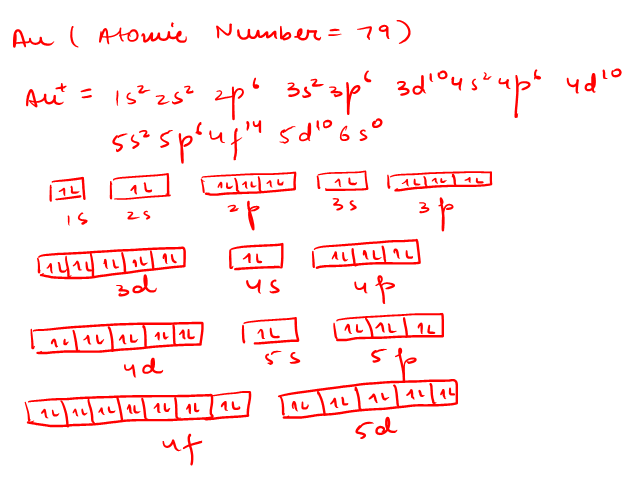
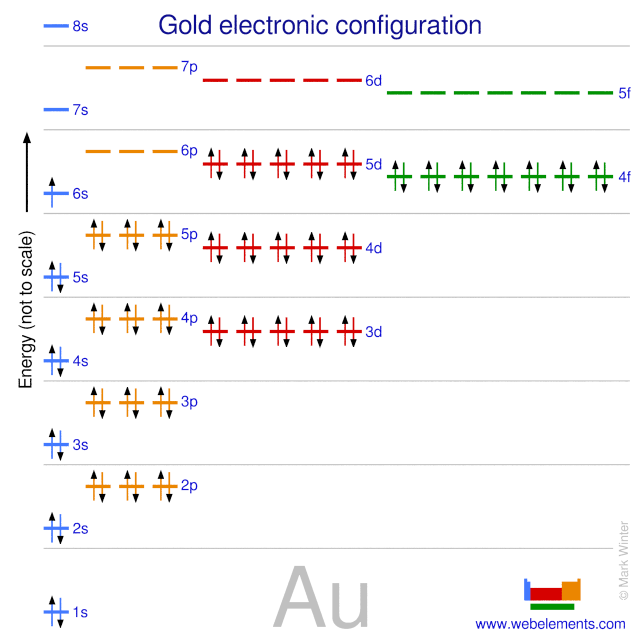
Write orbital diagram for Au+? - Soetrust Write orbital diagram for Au+? HERE THE ANSWERS. orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. you just have to fill the "boxes" with arrows, s orbital has only one box with two arrows; p has 3orbitals with 6arrows; d has 5 boxes with 10 arrows and f has 7boxes with 14 arrows. Write orbital diagram for Au+? 3 Answers. orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. you just have to fill the "boxes" with arrows, s orbital has only one box with two arrows; p has 3orbitals with 6arrows; d has 5 boxes with 10 arrows and f has 7boxes with 14 arrows. In each box can be two arrows with opposite spin maximum!
Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...

Write orbital diagram for au+.
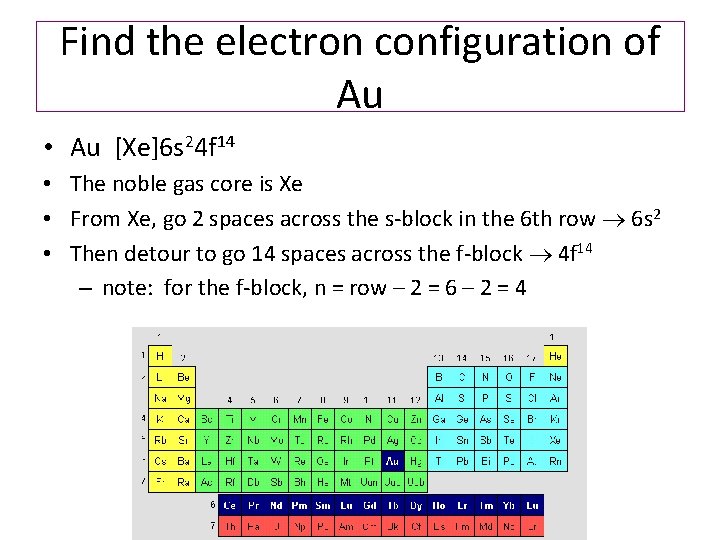
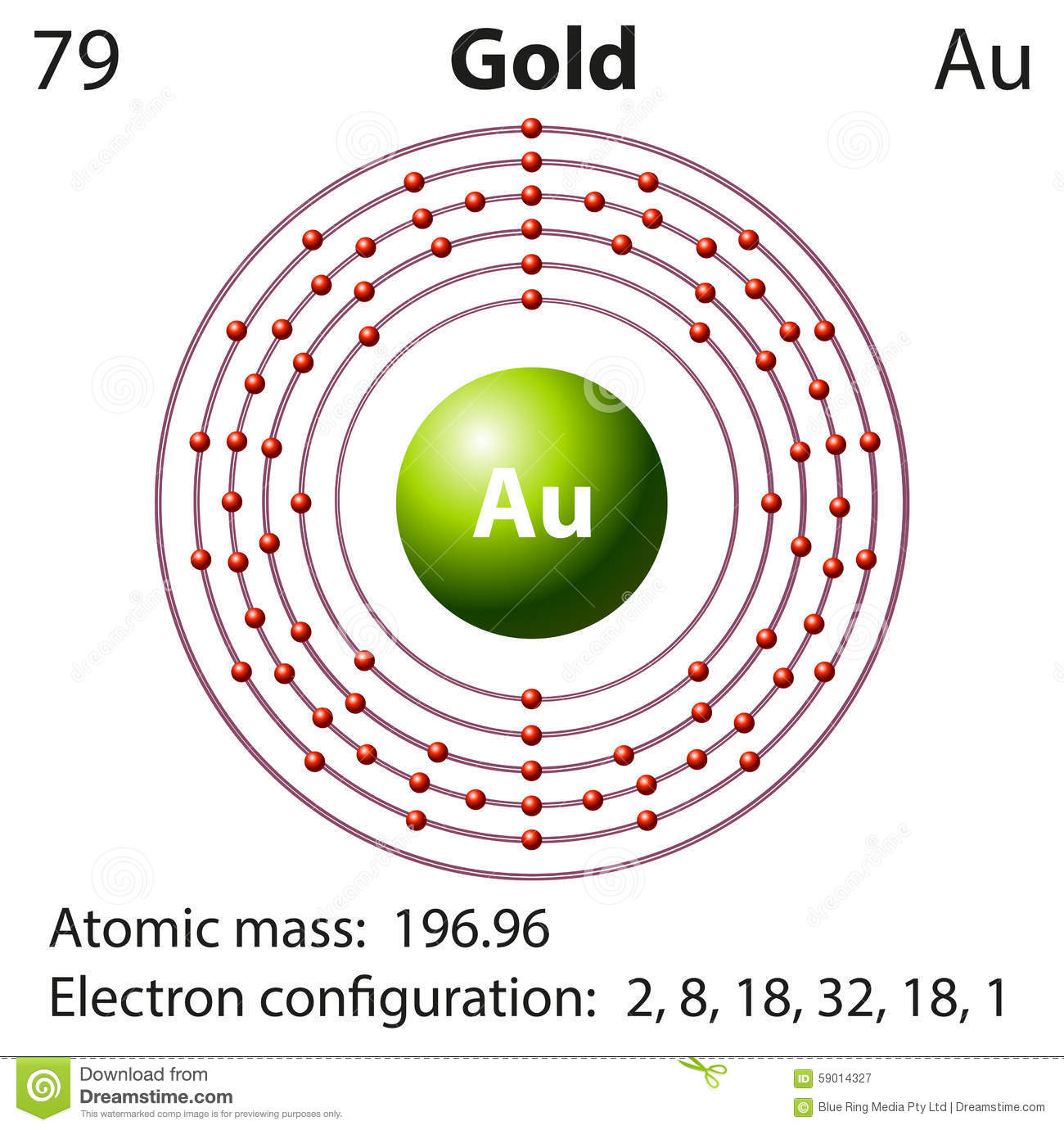
Draw the orbital diagram for Au+. | Study.com Molecular Orbital: Graphical representation of a molecule is done by following three rules, the Aufbau Principle, Hund's rule and the Pauli-Exclusion principle ...1 answer · Top answer: The give cation is Au+Au+ named as gold ion. The atomic number in periodic table is 79. Electronic configuration... SOLVED:Periodic Properties of the Elements | Chemistry ... Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ c. Mo3+ d. Zr2+ Lyniesha W. Numerade Educator View. Problem 79 Which is the larger species in each pair? a. Li or Li+ b. I- or Cs+ c. Cr or Cr3+ d. O or O2- ... What is the electron configuration of Au+? | Socratic 3 Jan 2016 — [Xe] 4f^14 5d^10 The atomic number of Au is 79. Therefore, its configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6 ...1 answer · [Xe]4f145d10 Explanation: The atomic number of Au is 79. Therefore, its configuration is: 1s22s22p63s23p63d104s24p64d105s25p64f145d106s1 or, [Xe]4f145d106s ...
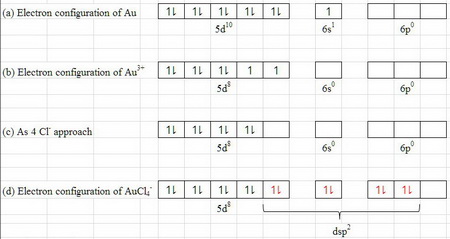
Write orbital diagram for au+.. Enter The Orbital Diagram For The Ion Mo3+. Answer to Write orbital diagram for Mo3+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing.write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd 2+ diagramweb.net + diagramweb.net 3+ d. Zr 2+ Provide your answer: example:paramagnetic, diamagnetic, etc., . What is the electron configuration of Au + ? - Toppr Click here to get an answer to your question ✍️ What is the electron configuration of Au + ?1 answer · Top answer: The atomic number of Au is 79.Therefore, its configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^25p^6 4f^14 5d^10 6s^1 or [Xe]4f^145d^106s^1 ... Excercise 14.13 question d - CHEMISTRY COMMUNITY Write the half-reactions, the balanced equation for the cell reaction, and the cell diagram for each of the following skeletal equations: d. Au+ (aq)--- Au (s)+Au3+ (aq) in this reaction, the oxidation half equation should be Au+ (aq)---Au3+ (aq)+2e-, but the answer on the back of the book gives Au (aq)---Au3+ (aq)+3e-. Enter the orbital diagram for the ion au+. Part 1. Hence, the orbital diagram for ion is as follows: Part 2. Therefore, cation is diamagnetic in nature. Ground state electron configuration for Au: 7. Au : 1s%2s²2p3s²3pº3d" 4s²4p 4d' 5s-5p4f"50"6s' Excited state electron configuration for Au* ion : ,Aut : 1s²2s²2p%3s 3p%3d" 4s24pº4d"°58²5p®48'45d".
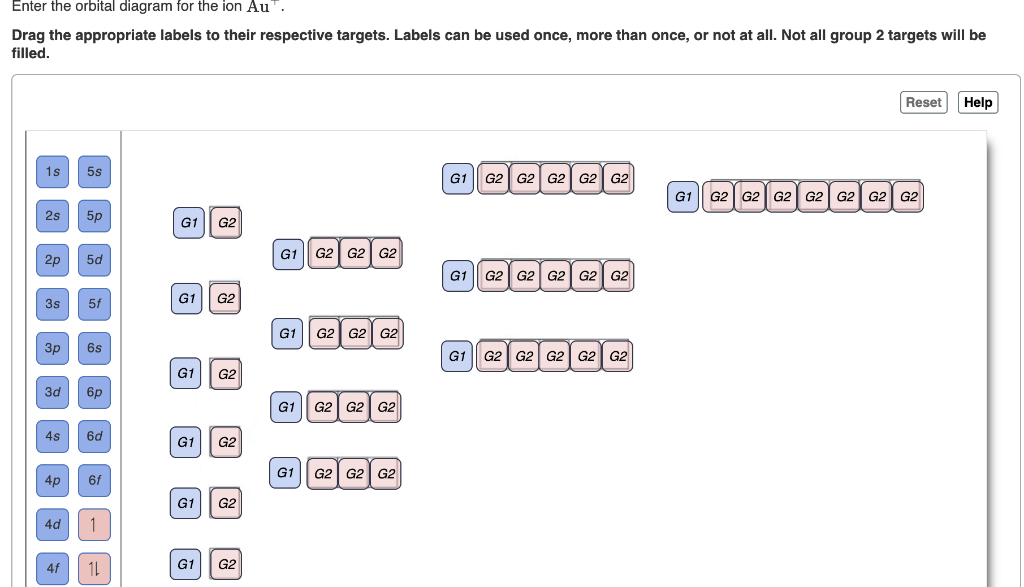
40 enter the orbital diagram for the ion cd2+. - Diagram ... Not all group 2 targets will be filled. Enter the orbital diagram for the ion Au+. Drag the appropriate labels to their respective targets. Write Orbital Diagram For Cd2+. - schematron.org Jan 09, 2018 · Question: Write orbital diagram for Cd2+ Use the buttons at the top of the tool to add orbitals. Write orbital diagrams for each ion and indicate whether ... Write orbital diagrams for each ion and indicate whether the | Quizlet. Answered: Write orbital diagrams for each ion and… | bartleby Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic.a. Cd2 + b. Au+ c. Mo3 + d. Zr2 + arrow_forward. Choose the more metallic element from each pair.(a) Sr or Sb (b) As or Bi(c) Cl or O (d) S or As. arrow_forward. Choose the more metallic element from each pair.a. Sr or Sb b. ... (Get Answer) - Write orbital diagram for Au+. Determine if ... Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. Posted one year ago. Q: Write the abbreviated electron configuration and construct the orbital diagram for the chromium(II) ion. Is the ion paramagnetic or diamagnetic? ...
Solved write orbital diagram for each ion and determine if ... write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd 2+ b.Au + c.Mo 3+ d. Zr 2+ Provide your answer: example :paramagnetic, diamagnetic, etc., accordingly to a, b, c,and d. zr2+ electron configuration To write the configuration for the Nickel ions, first we need to write the electron configuration for just Nickel (Ni). 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. Question: A) Write orbital diagram for Au+. One electron is removed from the outermost 6s orbital making the configuration xe 4f14 5d10. Shop with us is cr3+ paramagnetic or diamagnetic. SOLVED:Write orbital diagrams for each ion and determine ... Problem 78 Hard Difficulty. Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ c. Mo3+ d. Zr2+ (Get Answer) - Write orbital diagram for Zr2+. Write ... 1. Enter the orbital diagram for the ion Cd2+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons. 2. Enter the orbital diagram for the ion Au+. Use the...
Molecular orbital diagram of h2 - Soetrust Construct the molecular orbital diagram for he2; Use the molecular orbital diagram shown to determine which… using the molecular orbital theory, describe the bonding in… Write orbital diagram for Au+? Decide if N2 and N2+ are paramagnetic or diamagnetic. Which… What is the bond order of C2 2- ?
Answered: Write orbital diagrams for each ion and… | bartleby Solution for Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic.a. Cd2 + b. Au+ c. Mo3 + d. Zr2 +
Orbital Diagram Au+ the atomic number of au is therefore, its for au+, one electron is removed from the outermost 6s orbital, making the configuration. orbital diagram for au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the "boxes" with arrows, s orbital.orbital diagrams of atoms diagram shows how the electrons are distributed …
Write orbital diagram for Au+? - TheBasicAnswers.com answered May 20, 2021 by patel. selected May 20, 2021 by patel. Best answer. orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. you just have to fill the "boxes" with arrows, s orbital has only one box with two arrows; p has 3orbitals with 6arrows; d has 5 boxes with 10 arrows and f has 7boxes with 14 arrows.
Enter The Orbital Diagram For The Ion Au+., Write Orbital ... You are watching: Enter the orbital diagram for the ion au+. This is a memory device to remember the order of orbitals for the first two quantum numbers. Follow the arrow starting in the upper right, when the arrow ends go to the next arrow and start again.
Solved Write orbital diagram for Au+. Determine if the ion ... This problem has been solved! Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
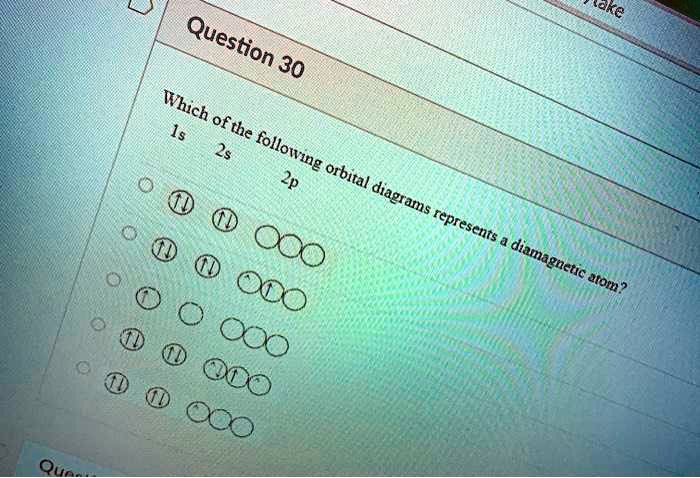
what subshell contains only one orbital? | page 3 write the orbital notation for the following set of quantum numbers n=1, l=0, m=0 . Physics. A polar satellite is launched at 850km above earth.Find its orbital speed. Chemistry. Use the drawing of a molecular orbital energy diagram for ClF to predict the bond order? CHEMISTRY. What atomic orbital has the quantum numbers n = 3, l = 1, ml = -1?
Orbital Diagram For Au+ - Wiring Diagrams Answer to Write orbital diagram for Au+. Draw an Molecular Orbital energy diagram and predict the bond order of L 2. Using a partial orbital diagram, show . If playback doesn't begin shortly, try restarting your device. Videos you watch may be added to the TV's watch history and influence TV recommendations.
Orbital Diagram For Au+ - schematron.org Figure Write orbital diagram for Au+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons. Jul 21, · Write orbital diagram for Au+ Determine if the ion is diamagnetic or paramagnetic. please helpStatus: Resolved.what is the orbital diagram for Au+, how do you fit the f orbitals in?what is the orbital diagram for Au , how do you fit the f orbitals in?

67. Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. ...
Chapter 8 Chemistry Homework Flashcards - Quizlet ANSWER: 1s2 2s2 2p6 3s2 3p6 4s2 4p6 4d10. Enter the orbital diagram for the ion Au+. ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed from the "s" sub-level. 1.) Remove one electron from 5s1. ANSWER: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10.
write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic a cd2 b au c mo3 d zr2
What is the electron configuration of Au+? | Socratic 3 Jan 2016 — [Xe] 4f^14 5d^10 The atomic number of Au is 79. Therefore, its configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6 ...1 answer · [Xe]4f145d10 Explanation: The atomic number of Au is 79. Therefore, its configuration is: 1s22s22p63s23p63d104s24p64d105s25p64f145d106s1 or, [Xe]4f145d106s ...
SOLVED:Periodic Properties of the Elements | Chemistry ... Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ c. Mo3+ d. Zr2+ Lyniesha W. Numerade Educator View. Problem 79 Which is the larger species in each pair? a. Li or Li+ b. I- or Cs+ c. Cr or Cr3+ d. O or O2- ...
Draw the orbital diagram for Au+. | Study.com Molecular Orbital: Graphical representation of a molecule is done by following three rules, the Aufbau Principle, Hund's rule and the Pauli-Exclusion principle ...1 answer · Top answer: The give cation is Au+Au+ named as gold ion. The atomic number in periodic table is 79. Electronic configuration...




















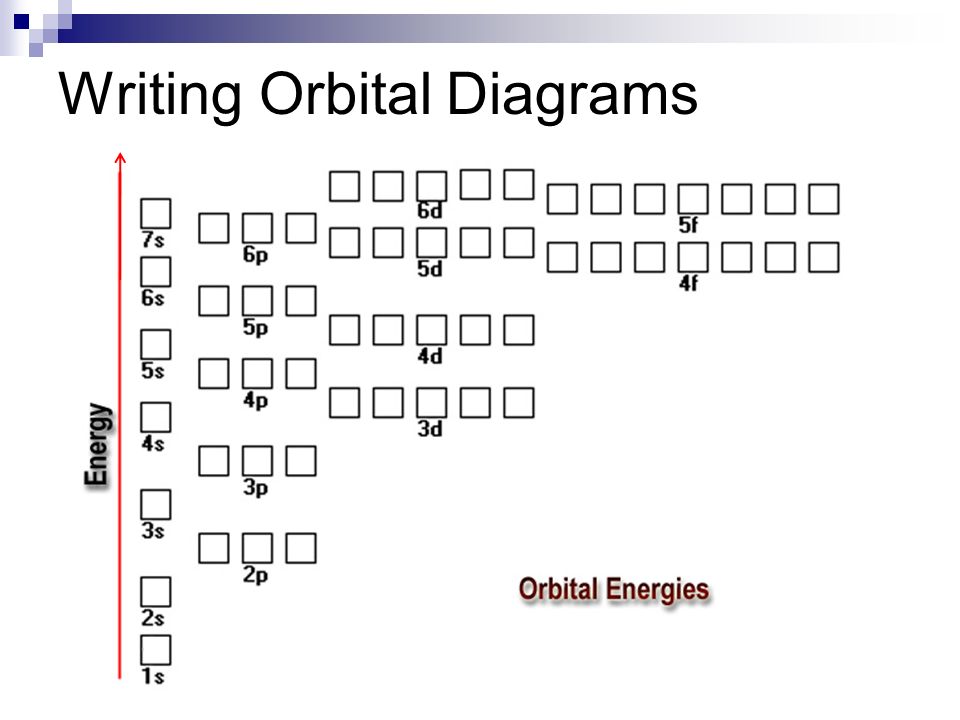
0 Response to "40 write orbital diagram for au+."
Post a Comment