39 lewis diagram for co
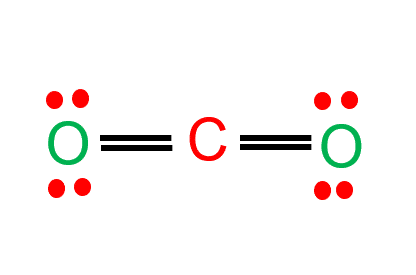
CO2 Lewis Structure - Easy Hard Science CO2 Lewis Properties. The CO 2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar. CO 2 is a nonpolar substance, meaning it tends to be a gas. CO 2 has a rather low boiling point of around -80 ℃ or -100 ℉. It can be liquified and even frozen solid with special machinery to produce "dry ice.". How Do You Draw The Lewis Structure For Co2 ... The Lewis structure of carbon dioxide (CO) consists of two oxygen atoms and one carbon atom. The carbon atom in the CO has two double bonds. The valence shells of carbon atoms and oxygen atoms each contain two lone pairs. Linearity is the characteristic of CO.
How to Draw the Lewis Dot Diagram for Carbon monoxide (CO ... te them around the central atom with the goal of filling the outer shells of each atom.In the Lewis structure of CO structure there are a total of 10 valence...

Lewis diagram for co
CO Lewis Structure - Lewis Dot Structure | Chem Helps CO Lewis Dot Structure. To write the CO Lewis Structure, we need to understand the formation of CO. CO, Carbon Monoxide has a total of 10 valence electrons, 4 of which are C and 6 are O. It consists of a carbon and an oxygen atom. A triple bond is formed between carbon and oxygen. 6 electrons form a triple bond. Lewis Structures: Learn How to Draw Lewis Structures ... How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16. Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element. CO2 Lewis Structure - Learnool CO 2 (carbon dioxide) has one carbon atom and two oxygen atoms. In the lewis structure of CO 2, there are two double bonds around the carbon atom, with two oxygen atoms attached to it, and on each oxygen atom, there are two lone pairs.
Lewis diagram for co. CO Lewis Structure, Geometry, and Hybridization ... CO Lewis Structure, Geometry, and Hybridization. Carbon monoxide (CO) is a tasteless and odorless flammable gas that is quite toxic in nature to the fauna. It is so because, carbon monoxide uses hemoglobin, an oxygen carrier, to reach throughout the body when in a concentration of more than 35ppm. The carbon monoxide is produced from the ... PDF LEWIS DIAGRAMS - Colorado State University Lewis diagrams, you would fi nd that the triangular form for ozone works well but it is excluded by experimental evidence. Therefore we must procede to work out the linear alternative: There are a total of 6 x 3 = 18 valence electrons, of which 4 are used in the single bonds, leaving 14. By Carbon monoxide (CO) Molecule Lewis Structure CO lewis structure. In the lewis structure of carbon monoxide, both atoms have eight electrons in their valence shells. However, oxygen atoms has a +1 charge and carbon atom has a +1 charge. In next sections, we will draw CO lewis structure step by step. Steps of drawing lewis structure of CO molecule. There are guidelines (several steps) to ... Lewis Structures: Dot Symbols, Diagrams, Examples, Questions Lewis Structure of Carbon Dioxide ( C O 2). In ( C O 2), oxygen belongs to group 16 of the Periodic Table, and carbon belongs to group 14 of the Periodic Table. Hence, oxygen has 6 valence electrons, and carbon has 4 valence electrons. Step 1- In ( C O 2) we, have C = 4 × 1 = 4 valence electrons. O = 6 × 2 = 12 valence electrons.
Solved Draw the Lewis structure for CO. | Chegg.com Draw the Lewis structure for CO. Question: Draw the Lewis structure for CO. This problem has been solved! See the answer See the answer See the answer done loading. Draw the Lewis structure for CO. Best Answer. This is the best answer based on feedback and ratings. 100% (14 ratings) Previous question Next question. What is the Lewis Structure of CO? - Quora Answer (1 of 3): Usually shown as There are various ways of configuring the electrons. Formal charge Suggests that things may be more complicated. CO2 Lewis Structure | Lewis Dot Structure For Molecules ... Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure works on the octet rule, which means that all the atoms would have eight electrons in their valence shell except hydrogen. How to Draw the Lewis Dot Structure for CH4N2O / CO(NH2)2 ... A step-by-step explanation of how to draw the CH4N2O Lewis Dot Structure (Urea).For the CH4N2O structure use the periodic table to find the total number of v...
CO2 (Carbon dioxide) Lewis Structure and Shape Steps of drawing the lewis structure of CO 2 are explained in detail in this tutorial. CO 2 lewis structure and Shape. In the lewis structure of CO 2, you can see there are two double bonds around carbon atom. Each oxygen atom has two lone pairs and carbon atom does not have a lone pair. Also, there are no charges in oxygen atoms and carbon atom. Write the Lewis dot structure of CO molecule class 11 ... - Lewis dot structure or electron dot structure or sometimes also called as Lewis electron dot structure is the diagram which shows the bonding between the atoms of a molecule and the lone pair of electrons that may exist in the molecule. - Lewis structure shows each tom and its position in the structure of a molecule by making use of chemical ... CO2 Lewis Structure (2021 UPDATED) All You Need To Know CO2 or carbon dioxide is considered as acid or can even be called Lewis Acid. The resonance structure accepts lone pairs of electrons, but the three lone pairs of electrons are in the oxygen molecule. There are no lone pairs of electrons in CO 2. Although, when dissolved in water, CO 2 takes the form of carbonic acid using this formula. Lewis Structure for CO - UMD The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. YouTube. Wayne Breslyn. 407K subscribers.
CO2 Lewis Structure, Hybridization, Molecular Geometry ... CO2 Lewis Structure. The lewis structure of CO2 can be with some simple steps, but before that, it is important to understand lewis structure properly. So lewis structure generally gives us an idea about the nature of bonding and octet fulfillment of the atoms. According to the octet rule, an atom attains stability by fulfilling its octet.
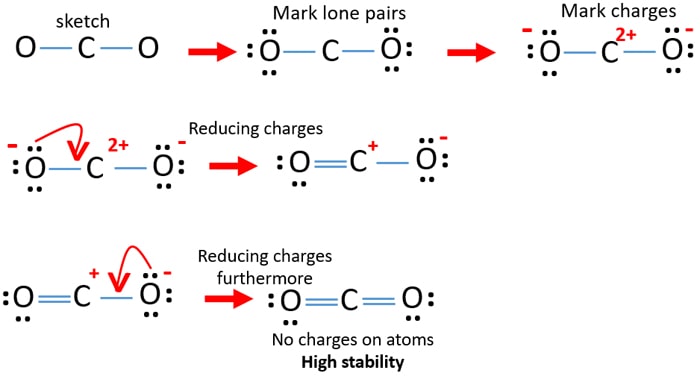
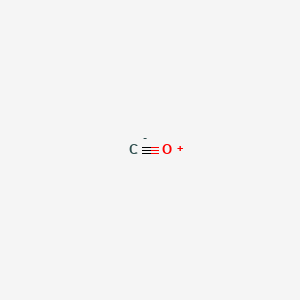
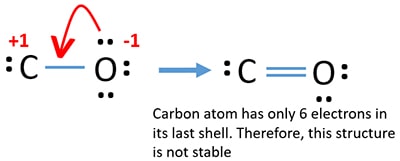
CO Lewis Structure - Learnool CO (carbon monoxide) has one carbon atom and one oxygen atom. In the lewis structure of CO, there is a triple bond between the carbon and oxygen atom, and on both carbon and oxygen atoms, there is one lone pair. Also, there is a negative (-1) charge on the carbon atom, and a positive (+1) charge on the oxygen atom.
Draw the lewis structure of co The Lewis dot structure of carbon monoxide that is CO needs to be drawn. Consider the valence electrons of each atom and distribute them in pair to form bonds between the atoms so as to satisfy the octet of each atom. The structure obtained is the required Lewis structure.
MO diagram and Lewis Structure of CO - CHEMISTRY COMMUNITY Re: MO diagram and Lewis Structure of CO Post by 104277942 » Fri Dec 06, 2013 7:36 am For bond order it is a good rule of thumb that triple bonds = 3 double = 2 and single = 1 but if you want to be sure, I personally like drawing a quick MO diagram sketch on the side and do the formula (bonding-antibonding)/2 to find the bond order if I am not ...
How can I draw the Lewis structure for CO? | Socratic Here are the steps that I follow when drawing a Lewis structure. 1. Decide which is the central atom in the structure. That will normally be the least electronegative atom (C). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: C-O. 3. Draw a trial structure by putting electron pairs around every atom until each gets an octet. In this editor, I will ...
CO Lewis structure, Hybridization, and Molecular Geometry ... CO Lewis structure, Hybridization, and Molecular Geometry (Carbon Monoxide) Carbon Monoxide is a colorless and odorless gas. This gas is less dense than the air and flammable. People know about this gas, as it can also cause poisoning. Carbon Monoxide is a toxic gas that binds with hemoglobin, which interferes with its binding with Oxygen.
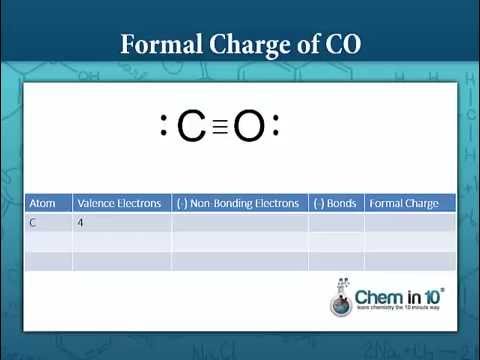
Write the Lewis dot structure of CO molecule. The lewis dot structure of carbon monoxide is: :C≡O: Solve any question of Chemical Bonding and Molecular Structure with:-.
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO 2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
How to Draw a Lewis Structure - ThoughtCo A Lewis structure is a graphic representation of the electron distribution around atoms. The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. A Lewis structure also helps to make a prediction about the geometry of a molecule.
CO Lewis Structure: How to Draw the Dot Structure for CO ... In the CO Lewis structure there aren't enough valence electrons available for each atom to obtain an octet without sharing more than one pair. Therefore CO has a triple bond between the carbon and oxygen atom. For the CO Lewis structure there are a total of 10 valence electrons available.
Draw the Lewis structure for CO - Ask4Essay The Lewis Structure for CO: 1. Count the electrons. 2. Put least electronegative atom in centre. 3. Put one electron pair in each bond. 4. Fill outer atoms with electrons. 5. Move electrons so all atoms has a full octet.
CO2 Lewis Structure - Learnool CO 2 (carbon dioxide) has one carbon atom and two oxygen atoms. In the lewis structure of CO 2, there are two double bonds around the carbon atom, with two oxygen atoms attached to it, and on each oxygen atom, there are two lone pairs.
Lewis Structures: Learn How to Draw Lewis Structures ... How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16. Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element.
CO Lewis Structure - Lewis Dot Structure | Chem Helps CO Lewis Dot Structure. To write the CO Lewis Structure, we need to understand the formation of CO. CO, Carbon Monoxide has a total of 10 valence electrons, 4 of which are C and 6 are O. It consists of a carbon and an oxygen atom. A triple bond is formed between carbon and oxygen. 6 electrons form a triple bond.



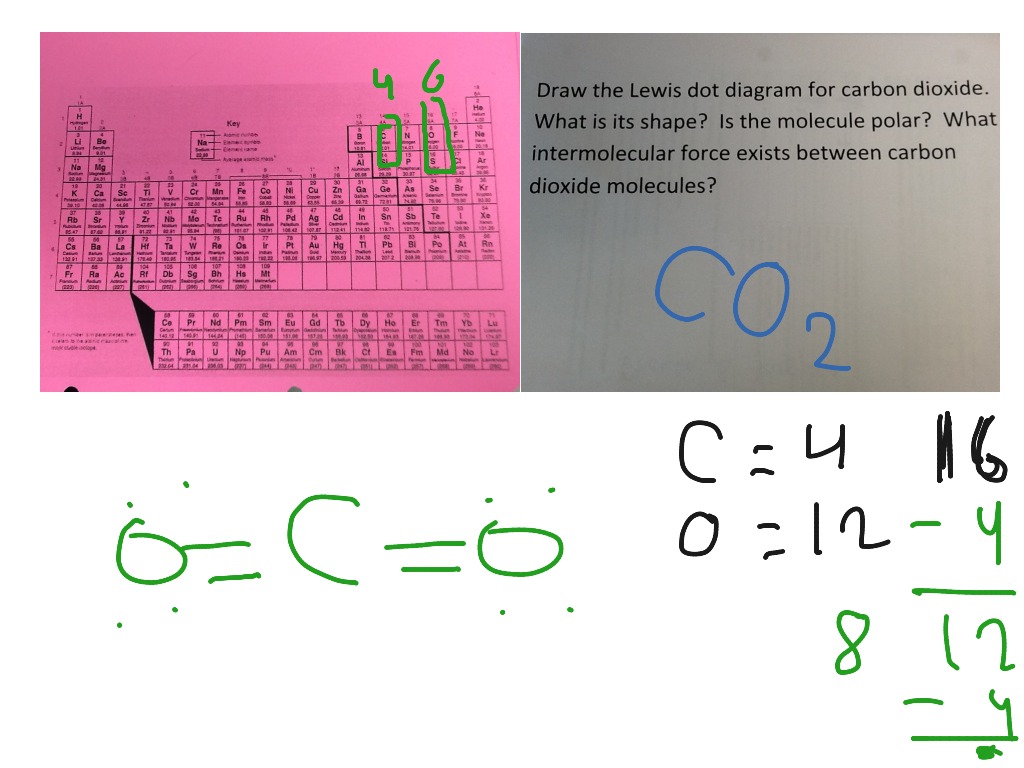

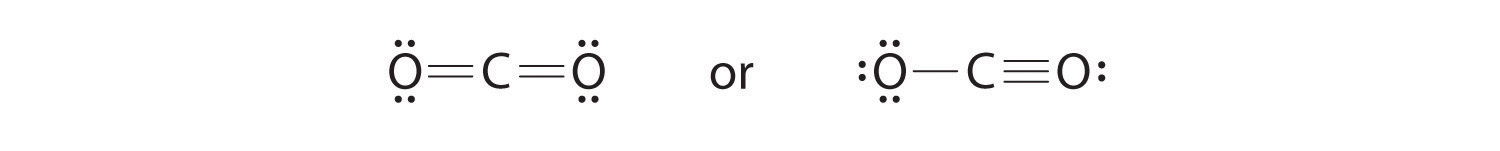
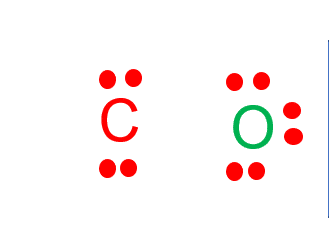
![Sample Paper Term 2] The table shows electronic structures of ...](https://d1avenlh0i1xmr.cloudfront.net/00ce2470-0895-455a-8597-a5192013f394/lewis-structure-of-co-01.jpg)


















0 Response to "39 lewis diagram for co"
Post a Comment