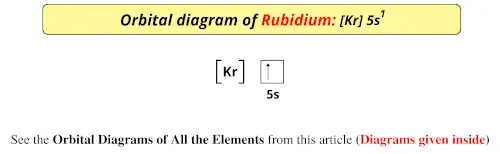
41 orbital diagram of potassium
Feb 18, 2021 · Just like there are 5 valence electrons for the element Vanadium. Similarly, every element will have its own valence electrons and many more. You can refer our article to those users or your friends who are looking for the information related to the Vanadium Electron Configuration of valence electrons as the good thing about our article is that it is available free of cost and no charges are ... Possible values for the magnetic quantum number m for a p orbital are -1, 0 and +1 (since ℓ is equal to one), which means that there can be three p orbitals in any of the electron shells except 1n. Once the s orbital in each electron shell has its complement of two electrons, the next six electrons will find a home in one of the p orbitals.
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line ... that the atom gains stability by having pairs of electrons in all its 3d orbitals but one unpaired electron in the 4s orbital. Potassium : atomic number (Z) = 19 (s block element) ...
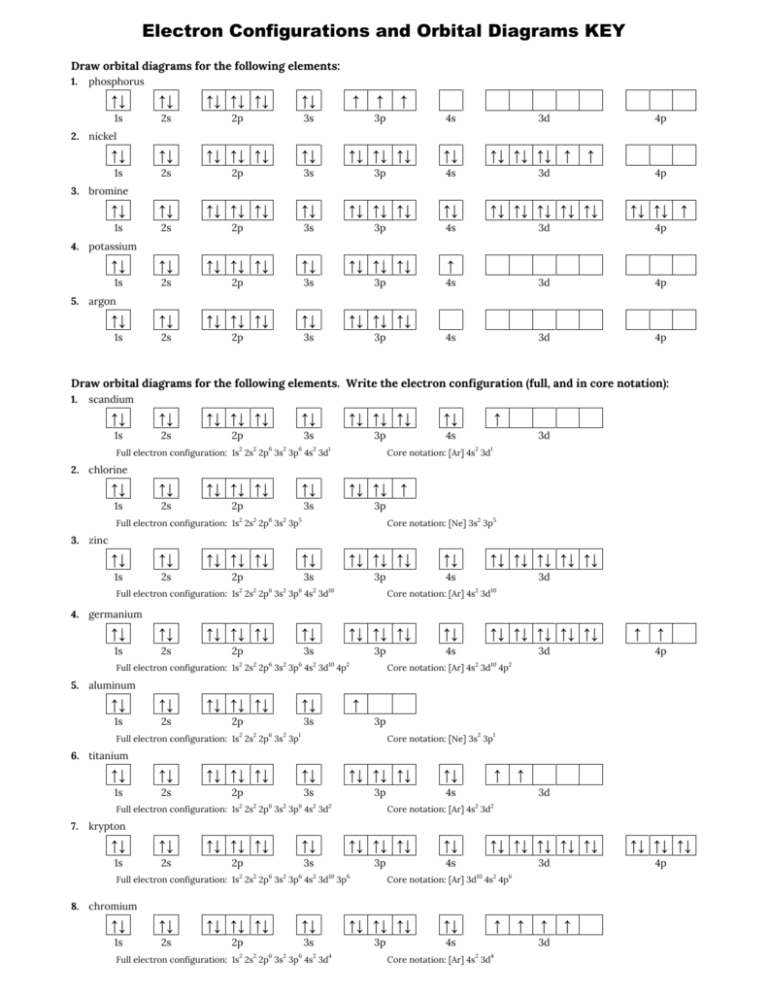
Orbital diagram of potassium
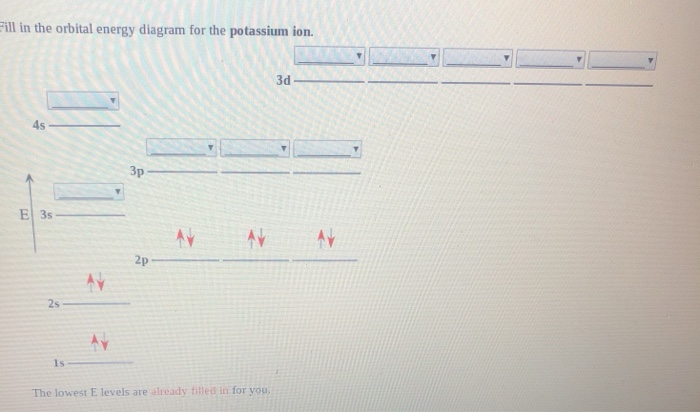
The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the ... Feb 15, 2021 · Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily. Chemistry questions and answers. ill in the orbital energy diagram for the potassium ion. 3d 4s 3p 3s 2p The lowest E levels are already fitted in for you.
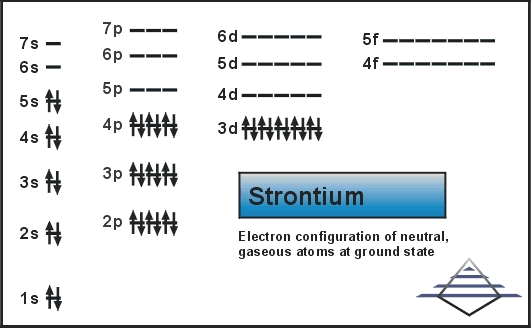
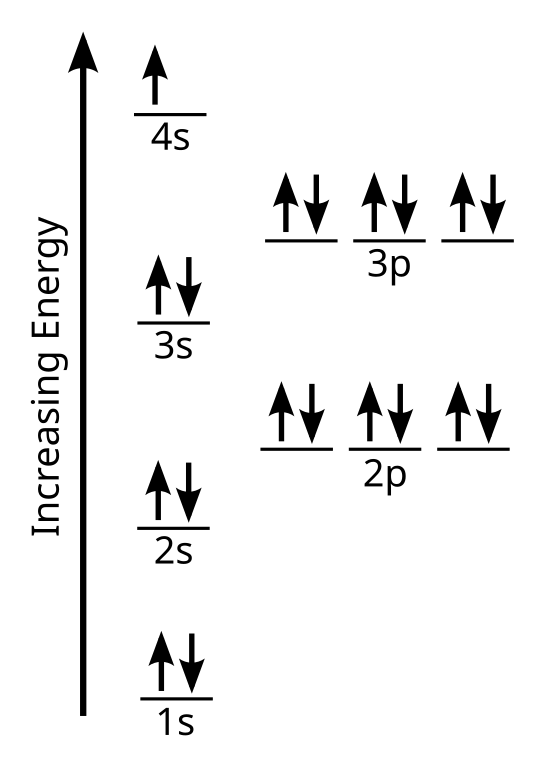
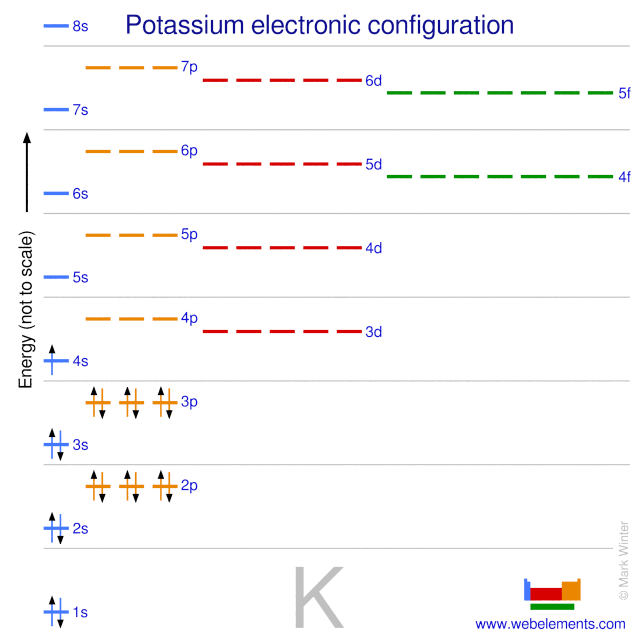
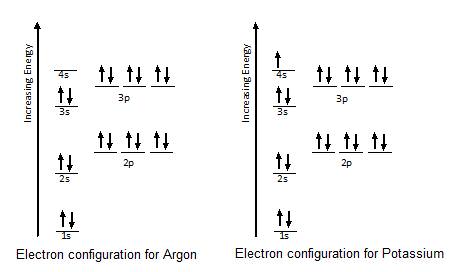
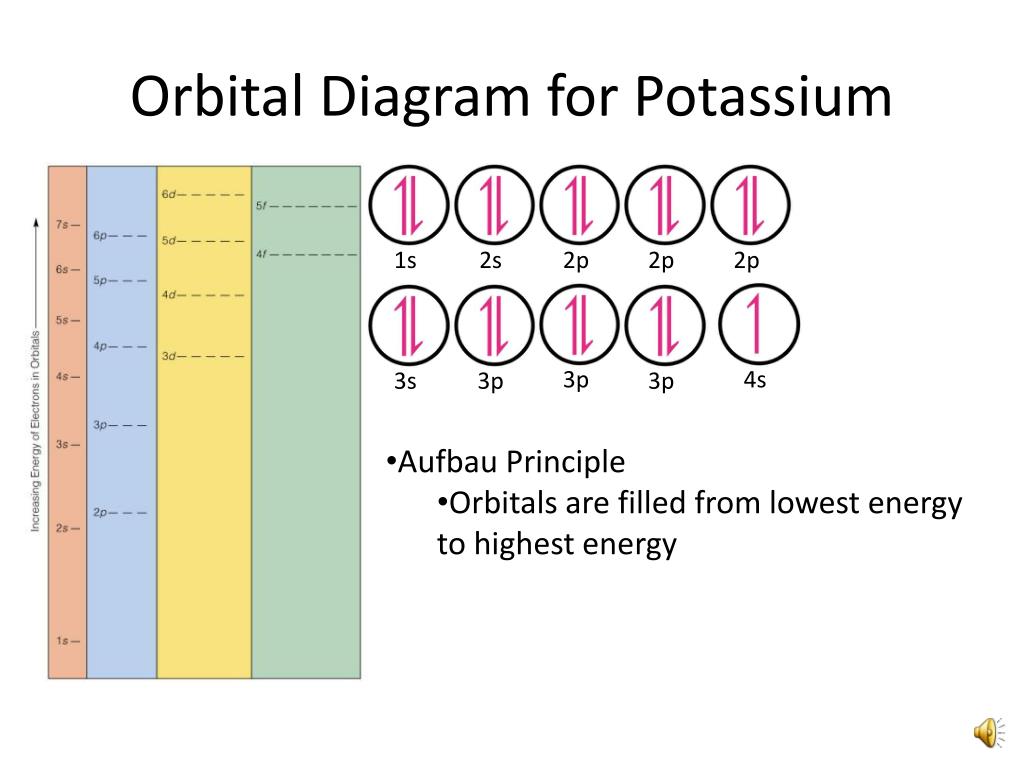
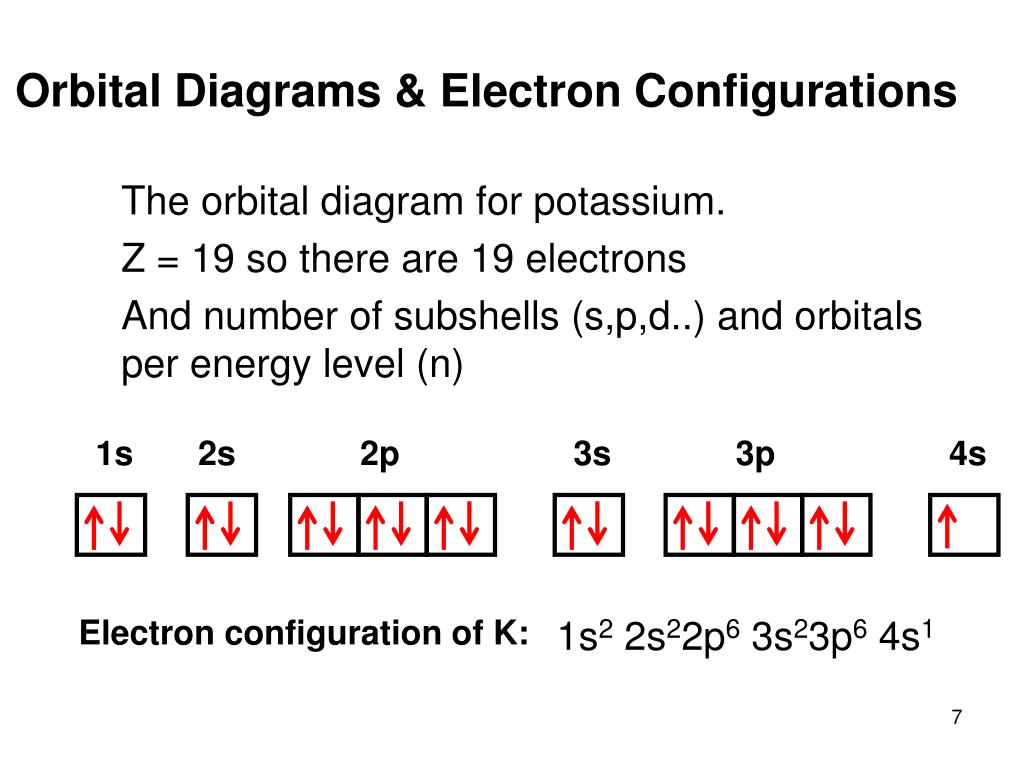
Orbital diagram of potassium. As discussed previously, the 3d orbital with no radial nodes is higher in energy because it is less penetrating and more shielded from the nucleus than the 4s, which has three radial nodes. Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. Orbital Diagram for Potassium. what is the electron configuration orbital diagram and potassium s atomic number is 19 this means that every atom of potassium has 19 protons in its nucleus in a neutral atom the number of protons is equal to the number of electrons so the electron configuration of potassium will involve 19 electrons the full electron configuration of potassium is "1s" 2"2s" 2"2p ... The energy level diagram below shows sublevels to as high as the energy level of the 5f orbitals. Sublevels actually continue to higher energies than this, but 5f is a suitable place to leave an introductory description. ... An s sublevel's orbital. Sublevel Examples ... Potassium's electron sublevels will be: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1. For ... Dec 17, 2014 · So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"4s"^1". The noble gas notation is "[Ar]4s"^1". The following orbital diagram shows the increase in energy from one energy sublevel to the next, but you can write them on the same level horizontally,
Milankovitch cycles describe the collective effects of changes in the Earth's movements on its climate over thousands of years. The term is named for Serbian geophysicist and astronomer Milutin Milanković.In the 1920s, he hypothesized that variations in eccentricity, axial tilt, and precession resulted in cyclical variation in the solar radiation reaching the Earth, and that this orbital ... que les valga pito. The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second ... So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is 1s2 2s2 2p6 3s2 3p6 4s1. The noble gas notation is [Ar]4s1. The orbital diagram shows the increase in energy from one energy sublevel to the next. The d electron count is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes. The formalism has been incorporated into the two major models used to describe coordination complexes; crystal ...
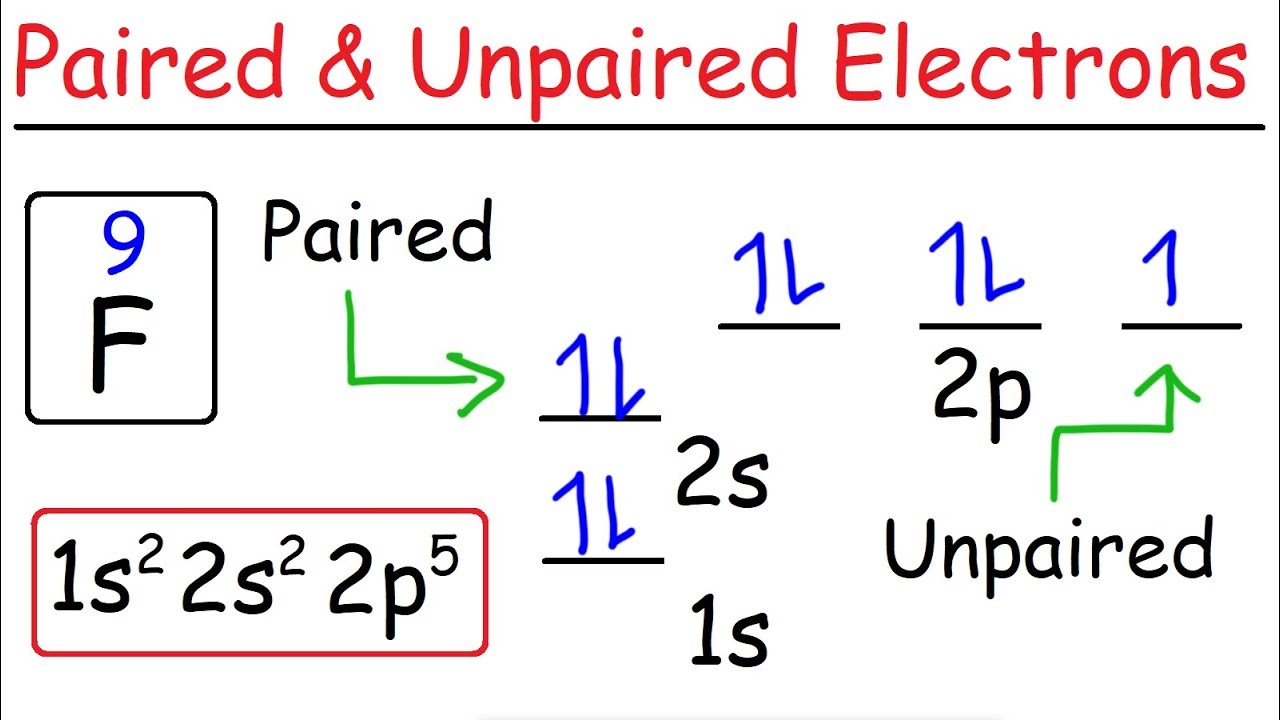
So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is 1s2 2s2 2p6 3s2 3p6 4s1. The noble gas notation is [Ar]4s1. The orbital diagram shows the increase in energy from one energy sublevel to the next. Orbital Diagram For Arsenic. Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! In writing the electron configuration for Potassium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Potassium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next ... The electron configuration of fluorine(F) and the orbital diagram is the main topic of this article. This article gives an idea about the fluorine orbital diagram, period and groups, valency and valence electrons of fluorine, bond formation, compound formation, application of different principles. The ninth element in the periodic table is ...
Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of ...
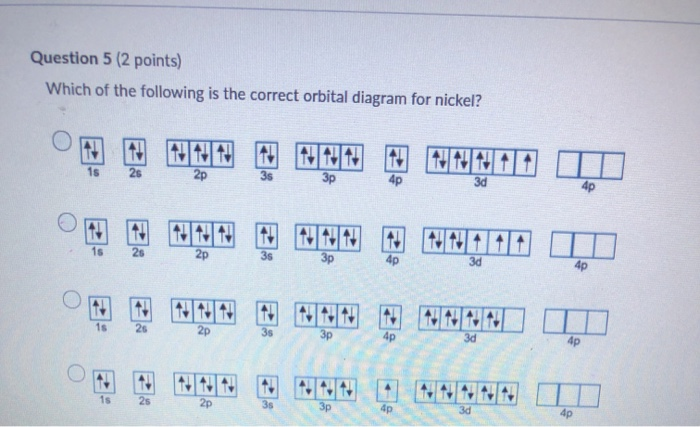
Chemistry questions and answers. ill in the orbital energy diagram for the potassium ion. 3d 4s 3p 3s 2p The lowest E levels are already fitted in for you.
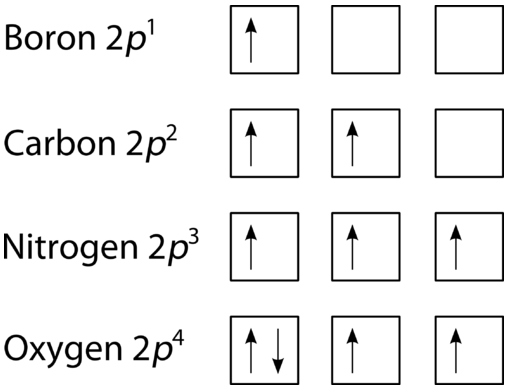
Feb 15, 2021 · Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily.
The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the ...





















0 Response to "41 orbital diagram of potassium"
Post a Comment