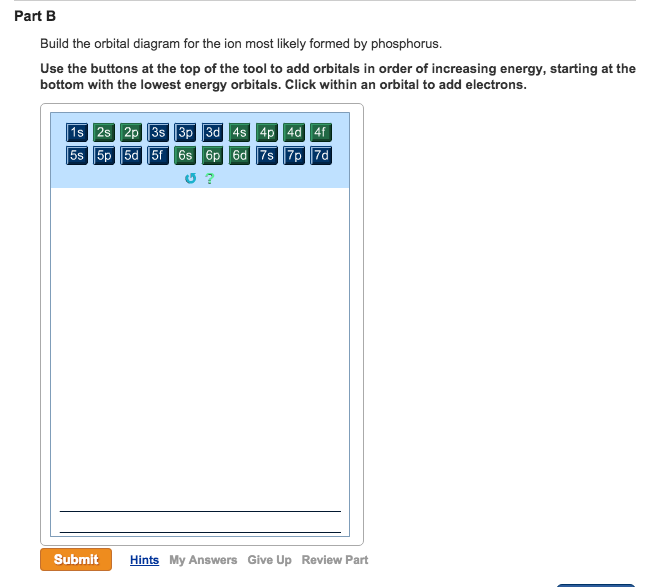
39 build the orbital diagram for the ion most likely formed by phosphorus
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. of an element to its ion? A. A uorine atom forms a F1 ion by losing one electron. B. A sodium atom forms a Na+1 ion by losing two electrons. C. A magnesium atom forms a Mg+2 ion by gaining two electrons. D. A phosphorus atom forms a P3 ion by gaining three electrons. 7. Which elements have the same number of neutrons? A. 10 5 B and 12 6 C B. 55 ...
sp 2 Hybridization. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital. This arrangement results from sp 2 hybridization, the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry ().
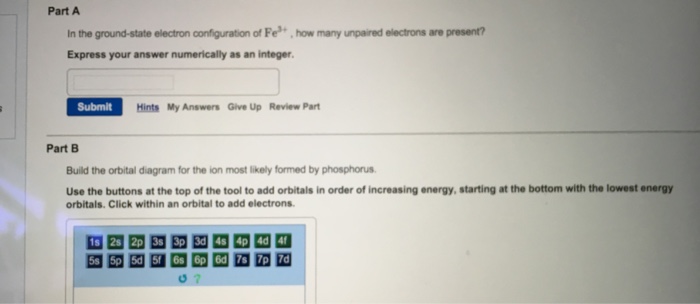
Build the orbital diagram for the ion most likely formed by phosphorus
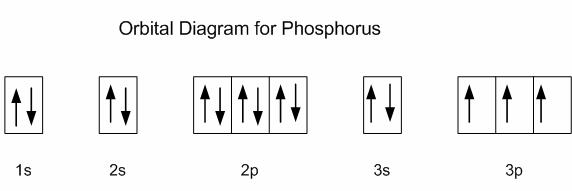
phosphorus - phosphorus - Properties and reactions: The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. The outer shell arrangement therefore resembles that of nitrogen, with three half-filled orbitals each capable of forming a single covalent bond and an additional lone-pair of electrons. Depending on the electronegativity of the elements with which it ... Orbital Diagram. The orbital diagram is a way of showing how the electrons are arranged in the atomic orbital of an atom or an ion by placing the electrons (arrows with up or down spins) in lines ... The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 3.
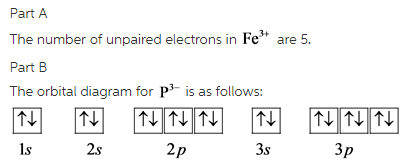
Build the orbital diagram for the ion most likely formed by phosphorus. Build the orbital diagram for the ion most likely formed by phosphorus? Build the orbital diagram for the ion most likely formed by phosphorus. Does anyone know what it is and how to answer it on mastering chemistry, that **** is so confusing. for mastering chem make sure the lower numbers r at the bottum. The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 5.1. 3 or 5.1. 4 ). Thus, the electron configuration and orbital diagram of lithium are: Build the orbital diagram for the ion most likely formed by phosphorus? 1s22s22p63s23p3 is for Phosphorus and the most likely ion is to be a 3- because it wants to have a full outer shell ... Draw orbital diagram s for the following elements: 1. Build the orbital diagram for the ion most likely formed by phosphorus? 1s22s22p63s23p3 is for Phosphorus and the most likely ion is to be a 3- because it wants to have a full outer shell ...
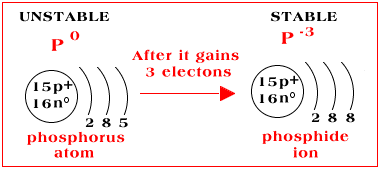
Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements. Part B:Build the orbital diagram for the ion most likely formed by phosphorus. Phosphorous forms an ion with a charge of -3, which means it has the same electron configuration as Argon. That electron configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6, Explain. What is the most likely ion for magnesium to become when it is ionized? Mg is more likely to become a cation with a 2+ charge by giving up 2 e- from the 3s sublevel. Mg more readily gives up these two e- thus requiring low IE to remove them. However, once the two 3s e- are removed, then Mg has an electron configuration of a noble gas (Ne). Build the orbital diagram for the ion most likely formed by phosphorus. Use the buttons at the top of the tool to add sublevels. Click within an orbital to add electrons. Question: Build the orbital diagram for the ion most likely formed by phosphorus. Use the buttons at the top of the tool to add sublevels. Click within an orbital to add ...
Build the orbital diagram for the ion most likely formed by phosphorus. ... In which orbital does an electron in a phosphorus atom experience the greatest effective nuclear charge? a. 1s b. 2s c. 2p d. 3s e. 3p I originally thought 3p, since those are the outer electrons, but that was marked wrong. ... Use the drawing of a molecular orbital ... Build the orbital diagram for the ion most likely formed by phosphorus? Wiki User. ∙ 2014-11-24 20:57:15. See Answer. Best Answer. Copy. 1s22s22p63s23p3 is for Phosphorus and the most likely ion ... Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 2p^4# F #1s^2 2s^2 2p^5# All Group 17 Elements (halogens) gain one electron to form an ion with a 1- charge e. All Group 16 nonmetals gain two electrons to form an ion with a 2- charge f. All Group 15 nonmetals gain three electrons to form an ion with a 3- charge Notice that cations keep their name (sodium ion, calcium ion) while anions get an "-ide" ending
Click wtihin an orbital to add electrons. Question: Part BBuild the orbital diagram for the ion most likely formed by phosphorus. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals. Click wtihin an orbital to add electrons.
Build the orbital diagram for the ion most likely formed by phosphorus. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, m s = + 1 2 ).
Select the charge and write the full ground-state electron configuration of the monatomic ion most likely to be formed by Rb. Charge: a. +2 b. +1 c. 0 d. -1 e. -2 Ground-state electron confi ...
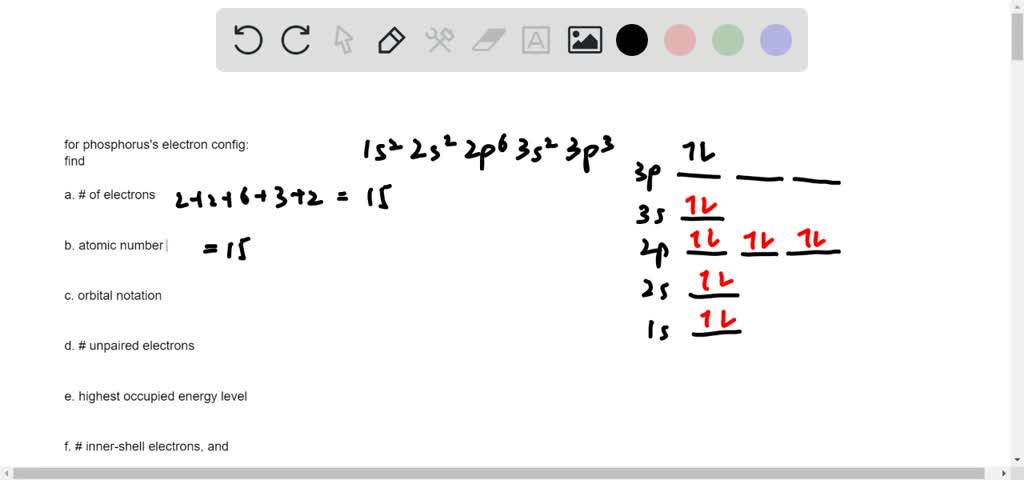
We're asked to build the orbital diagram for the ion most likely formed by phosphorus. This requires determining first the ground-state electronic configuration of phosphorus (P) by referring to the periodic table and locating the position of P. Ground state means that the element is in its lowest energy form (not in an excited state).
Box spin diagram of outer electron orbitals for the electron configuration of the 15 Phosphorus, P, 1s22s22p63s23p3 (), [Ne]3s 3p, P, p-block, Gp5/ Build the orbital diagram for the ion most likely formed by phosphorus? a \ will be an arrow going one way and a / will be the other way. a [ ] represents a box. Phosphorus atomic orbital and ...
Build The Orbital Diagram For The Ion Most Likely Formed By Phosphorus. Orbital for ion most likely formed by phosphorus? Each element in the periodic table has a distinctive The more electrons that are lost, the smaller the ion becomes. more electrons gained, larger the ion becomes.
The Aufbau Principle. We construct the periodic table by following the aufbau principle (from German, meaning "building up"). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli principle.We use the orbital energy diagram of Figure \(\PageIndex{1}\), recognizing that each orbital can ...
5 Build the orbital diagram for the ion most likely formed by phosphorus. Electron Configurations of Atoms and Ions The electron configuration of an atom tells us how many electrons are in each orbital. For example, helium has two electrons in the 1s orbital. Therefore the electron configuration of He is 1s².
The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 3.
Orbital Diagram. The orbital diagram is a way of showing how the electrons are arranged in the atomic orbital of an atom or an ion by placing the electrons (arrows with up or down spins) in lines ...
phosphorus - phosphorus - Properties and reactions: The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. The outer shell arrangement therefore resembles that of nitrogen, with three half-filled orbitals each capable of forming a single covalent bond and an additional lone-pair of electrons. Depending on the electronegativity of the elements with which it ...









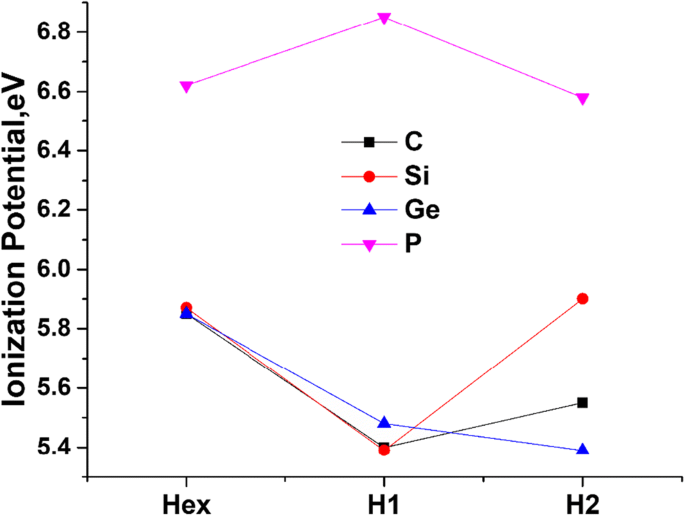







0 Response to "39 build the orbital diagram for the ion most likely formed by phosphorus"
Post a Comment